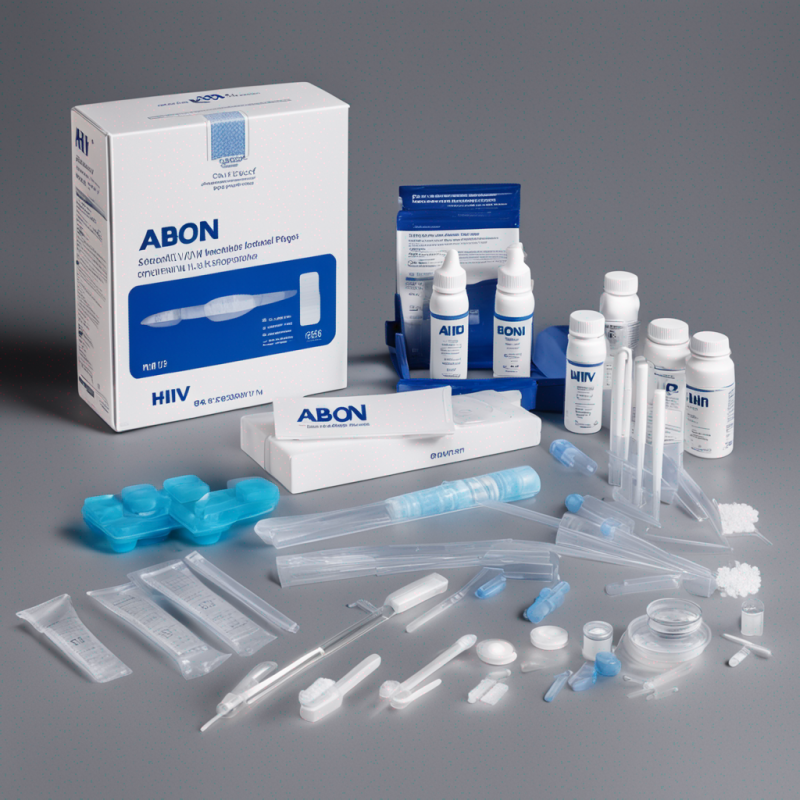
ABON HIV 1/2/O Tri-Line Kit: Advanced, Rapid HIV Detection Solution
ProcurenetShort description
ABON HIV 1/2/O Tri-Line Kit – Rapid and Precise HIV Antibody Detection
Effectively detect antibodies of HIV-1, HIV-2, and subtype O with this kit, powered by advanced rapid immunochromatographic assay technology. Key Features:
- Performs 40 tests per kit using whole blood, serum, or plasma samples.
- Provides results within 10 minutes with a sensitivity of 100% and specificity of 99.7%.
- Comprehensive Kit: Comes with test devices, specimen droppers, buffer, instructions, and recommends additional requirements.
- Has a 24-month shelf life when stored between 2 – 30°C.
Choose the ABON HIV 1/2/O Tri-Line Kit for precise and quick HIV testing.
-
Procurenet Team Tshim Sha Tsui
Hong Kong 3 years
Description
ABON HIV 1/2/O Tri-Line Kit: State-of-the-Art HIV Testing
Experience exceptional diagnostic accuracy with the ABON HIV 1/2/O Tri-Line Kit, a versatile and high-performing HIV testing solution. Combining state-of-the-art technology with robust testing methodologies, this kit offers a breakthrough solution for the detection of HIV-1, including subtype O, and HIV-2 antibodies.
Powered by the advanced rapid immunochromatographic assay technology, this kit separates itself from the competition, providing unmatched levels of sensitivity and specificity. This delicate balance ensures minimal risk of false negatives or positives, offering clinicians and patients alike peace of mind and confidence in results.
- Advanced Immunochromatographic Assay Technology: Rely on the ABON kit's high tech operating system for uniform and precise HIV-1 and HIV-2 antibody detection.
- Flexible Sampling Options: Adapt to any patient scenario with flexible sampling options. Use venous and capillary whole blood, serum, or plasma samples to run the test.
- Remarkable Sensitivity & Specificity: The ABON kit offers a remarkable sensitivity and specificity balance, boasting 100% sensitivity and 99.7% specificity, reducing false negatives and positives.
- Speed & Efficiency: Save time with efficient results delivery within just 10 minutes, enabling swift decision-making and effective patient management.
- Comprehensive Contents: Each kit arrives complete with pouched test devices with desiccant, specimen droppers, buffer, and easy-to-follow instructions.
- Long Shelf Life & Convenient Storage: The ABON kit has a 24-month shelf life and requires storage between 2 - 30°C, away from direct sunlight and high humidity, ensuring it continues to deliver optimal results.
Designed for precision and ease of use, the ABON HIV 1/2/O Tri-Line Kit is your go-to solution for rapid, reliable HIV testing. It is essential to use the kit as recommended by the manufacturer and in accordance with standard biosafety precautions.
For additional information or for details on the World Health Organization's list of prequalified in vitro diagnostic products, consult the manufacturer's instructions included in the kit.