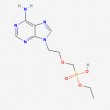
Adefovir Dipivoxil Impurity 16 - 10mg
Short description
Adefovir Dipivoxil Impurity 16
Discover the exceptional Adefovir Dipivoxil Impurity 16, a high-purity compound with a CAS number of 116384-54-4. This versatile chemical boasts a molecular weight of 301.24g/mol and a molecular formula of C10H16N5O4P, offering a unique blend of properties for your research needs. Handled with care, this product is intended for laboratory use only and should not be consumed. Unlock the potential of this premium-quality impurity in your next synthesis or analysis, and experience the reliability and precision that this exceptional compound delivers.
-
Procurenet Team Tshim Sha Tsui
Hong Kong 3 years
Description
Adefovir Dipivoxil Impurity 16
Adefovir Dipivoxil Impurity 16, with the CAS number 116384-54-4, is a highly specialized chemical compound that plays a crucial role in the research and development of pharmaceutical products. As an impurity associated with the active pharmaceutical ingredient (API) Adefovir Dipivoxil, this compound offers valuable insights into the quality and purity of the parent drug.
Adefovir Dipivoxil Impurity 16 has a molecular formula of C10H16N5O4P and a molecular weight of 301.24 g/mol. Its unique chemical structure and properties make it an essential tool for researchers and scientists working in the pharmaceutical industry. This impurity provides a deeper understanding of the synthesis, stability, and potential degradation pathways of Adefovir Dipivoxil, a widely used antiviral medication.
The presence and identification of Adefovir Dipivoxil Impurity 16 in pharmaceutical formulations are crucial for ensuring the safety, efficacy, and regulatory compliance of the final drug product. By studying this impurity, researchers can develop more robust analytical methods, optimize purification processes, and gain valuable insights into the overall quality control of Adefovir Dipivoxil-based therapies.
Analytical Applications
Adefovir Dipivoxil Impurity 16 serves as a valuable reference standard in the analytical characterization of Adefovir Dipivoxil and its related substances. Researchers can utilize this impurity to establish sensitive and reliable analytical methods, such as high-performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS), to detect, quantify, and monitor the presence of this impurity in drug samples.
The availability of Adefovir Dipivoxil Impurity 16 allows for the development of comprehensive impurity profiles, which are essential for regulatory submissions and ensuring the quality and safety of pharmaceutical products. By understanding the behavior and characteristics of this impurity, researchers can better predict and mitigate potential quality issues, ultimately contributing to the overall success of Adefovir Dipivoxil-based drug development and manufacturing.
Stability and Degradation Studies
Adefovir Dipivoxil Impurity 16 plays a crucial role in stability and degradation studies of Adefovir Dipivoxil. By studying the formation, behavior, and fate of this impurity under various stress conditions, such as heat, humidity, and oxidation, researchers can gain valuable insights into the stability and potential degradation pathways of the parent API.
These studies are essential for establishing appropriate storage conditions, shelf-life, and handling protocols for Adefovir Dipivoxil-based pharmaceutical products. The data generated from these investigations can also inform the development of more robust and reliable formulations, ensuring the long-term quality and integrity of the drug substance and drug product.
Regulatory Compliance
In the highly regulated pharmaceutical industry, the identification and control of impurities, such as Adefovir Dipivoxil Impurity 16, are critical for meeting regulatory requirements. Regulatory authorities, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have established guidelines and limits for the presence of impurities in drug substances and drug products.
By providing a well-characterized reference standard for Adefovir Dipivoxil Impurity 16, researchers can demonstrate compliance with these regulatory guidelines, ensuring the safety and efficacy of the final drug product. This, in turn, facilitates the successful submission and approval of Adefovir Dipivoxil-based pharmaceutical applications, ultimately benefiting patients in need of effective antiviral therapies.
Conclusion
Adefovir Dipivoxil Impurity 16 is a crucial component in the research and development of Adefovir Dipivoxil-based pharmaceutical products. Its availability as a reference standard enables researchers to conduct comprehensive analytical studies, stability assessments, and regulatory compliance evaluations
Specifications
- Formula: C10H16N5O4P
- Molecular weight: 301.24
- Notes: This product is intended for laboratory use only, and it is not meant for human consumption.





