Uni-Gold™ HIV Complete Kit: Rapid HIV-1 & HIV-2 Antibody Detection
ProcurenetShort description
Uni-Gold™ HIV Complete Kit: Rapid HIV-1 & HIV-2 Antibody Detection Kit
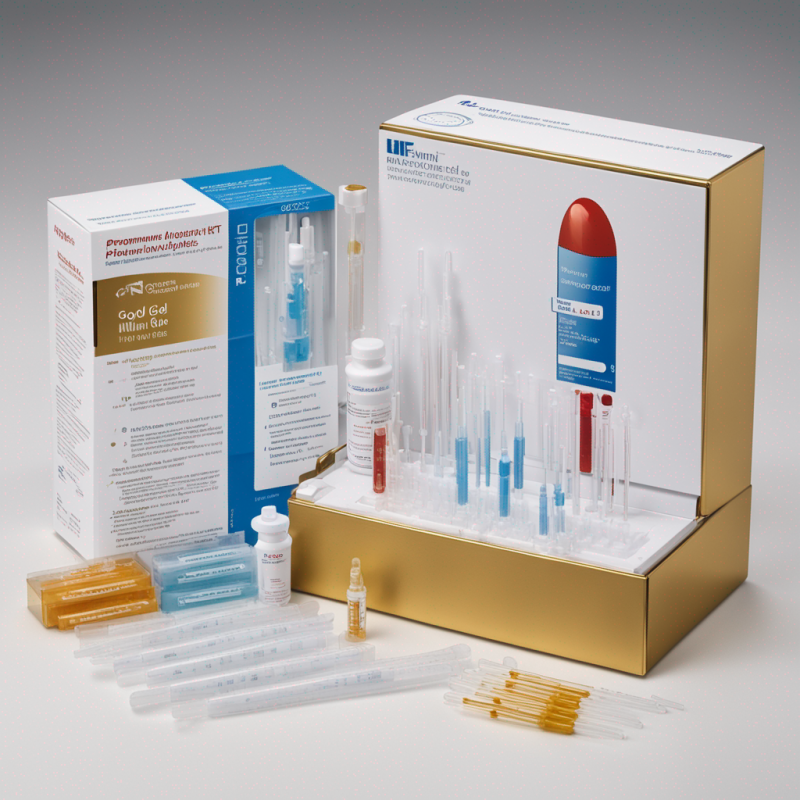
This is a high-sensitivity immunoassay kit that has been optimized to effectively detect HIV-1 and HIV-2 antibodies in serum, plasma or whole blood. The rapid immunochromatographic method enables results within a minimum of 10 minutes. Each kit contains 20 test devices, formula wash solution, disposable pipettes and lancets, and instructions.
- Sensitivity: High, at 99.76%
- Specificity: Excellent, at 99.85%
- Contents: Inclusive of Test devices, wash solution, disposable pipettes & lancets, and a guide
- Shelf Life: Reliable with 20 months at 2 to 27°C
- Result Time: Rapid, with a minimum of 10 minutes
Note: Additional requirements are present, such as blood collection devices, biohazard disposal container, sterile gauze pads, timer, and personal protection equipment. This kit is exclusively for diagnosis purposes, not suitable for donor screening.
-
Procurenet Team Tshim Sha Tsui
Hong Kong 3 years
Description
Uni-Gold™ HIV Complete Kit: Rapid HIV-1 & HIV-2 Antibody Detection Kit
The Uni-Gold™ HIV Complete Kit is a comprehensive and easy-to-use solution for fast and accurate detection of HIV-1 and HIV-2 specific antibodies in blood samples. This kit is designed to offer reliable results within 10 minutes, ensuring swift diagnostic reporting in critical health check situations.
Compelling Features:
- Fast Testing: A rapid immunoassay utilizing the immunochromatographic sandwich principle gives accurate results within 10 minutes.
- Convenient Use: The test procedure is user-friendly with easy-to-interpret results.
- Flexible Sample Types: It works with various sample types, including serum, plasma, and whole blood, thus providing versatility.
- High Accuracy: 99.76% sensitivity and 99.85% specificity ensure the reliability of the results.
Components of the Kit:
- 20 individually-packaged test devices for ensured hygiene.
- Wash solution in a dropper bottle for easy application.
- Disposable elements including 20 pipettes and sterile lancets.
- An in-depth instruction manual for detailed guidance.
Note on Storage and Shelf Life:
Keeping the unopened kits at 2 to 27°C will maintain their effectiveness. Their shelf life is well over 20 months.
Additional Requirements (not included in the kit):
- Blood collection devices for testing.
- A biohazard waste disposal container.
- Gauze pads, adhesive bandages for finger prick samples.
- A timer or stopwatch to keep track of the process time.
- Personal protective equipment for the safety of the users.
Usage Guidelines:
The kit's manual presents exhaustive instructions to conduct tests with high accuracy. The kit is purely diagnostic and not meant for screening blood, plasma, cells, or tissue donors.