TaqPath COVID-19 CE-IVD RT-PCR Kit: Superior SARS-CoV-2 Diagnostic Solution
ProcurenetShort description
TaqPath COVID-19 CE-IVD RT-PCR Kit for Accurate SARS-CoV-2 Detection
A diagnostic solution with exceptional effectiveness in qualitative detection of SARS-CoV-2 nucleic acid. Specifically designed for nasopharyngeal and lavage samples, this kit offers:
- Dependability: Proven accuracy and reliability in SARS-CoV-2 detection.
- Convenience: Comprehensive kit with necessary reagents and controls for seamless workflow.
- Robust Storage: Each component includes storage conditions to maintain optimal effectiveness.
Rigorously tested and validated, this kit provides consistent performance on Applied Biosystems real-time PCR systems. It has a shelf life of 12 months from the manufacture date.
-
Procurenet Team Tshim Sha Tsui
Hong Kong 3 years
Description
TaqPath COVID-19 CE-IVD RT-PCR Kit: Premier Diagnostic Resource for Accurate SARS-CoV-2 Detection
The TaqPath COVID-19 CE-IVD RT-PCR Kit stands as an exceptional, precision-crafted diagnostic solution, offering qualitative detection of SARS-CoV-2 nucleic acid in suspected COVID-19 patients. This premier tool is specifically engineered to be fully compatible with Applied Biosystems real-time PCR systems, providing reliable detection, thereby serving as a key asset in diagnosis and control of the pandemic.
- Expertly engineered for reliable and accurate SARS-CoV-2 nucleic acid detection
- Optimized for seamless integration with Applied Biosystems real-time PCR systems
- Includes all vital reagents and controls for the testing process
- Verified and endorsed for performance evaluation with nasopharyngeal swabs, nasopharyngeal aspirates, and bronchoalveolar lavage samples
- Strict adherence to recommended procedures for collection, transportation, and storage ensures accurate results
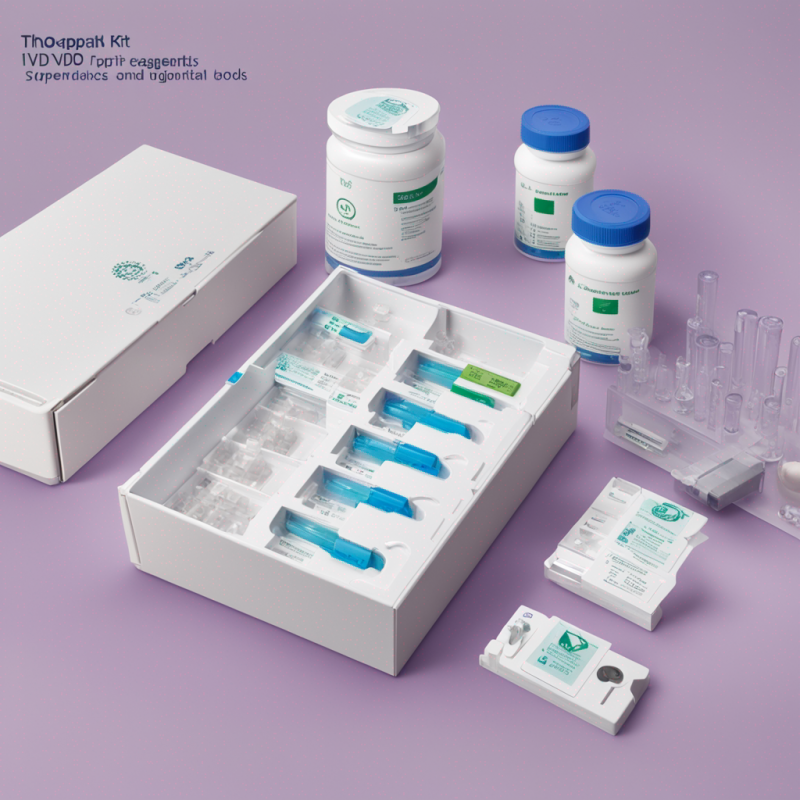
- A comprehensive kit that features TaqPath COVID-19 RT-PCR Kit, MS2 Phage Control, TaqPath COVID-19 Control, TaqPath COVID-19 Control Dilution Buffer, and TaqPath 1-Step Multiplex Master Mix (No ROX)
- Accompanied by easy-to-follow reference insert
- Free installation and warranty repairs for the first year
Technical Specs
This kit’s prowess lies in its meticulously selected ingredients and robust technology. Notably, specimen types other than nasopharyngeal swab, nasopharyngeal aspirate, and bronchoalveolar lavage samples should not be used with this assay as they haven’t been tested.
Recommended Instruments/Systems:
- Applied Biosystems 7500 series, including but not limited to Fast Dx RealTime PCR Instrument, Fast RealTime PCR Instrument, and RealTime PCR Instrument
- QuantStudio 5 series, with instruments such as RealTime PCR Instrument for distinct well blocks and the Dx RealTime PCR Instrument
- QuantStudio 7 Flex Real-Time PCR Instrument, for 384-well block, utilized with QuantStudio RealTime PCR Software v1.3
Storage and Shelf Life
The contents of the kit necessitate specific storage conditions to ensure optimal performance. Guaranteed shelf life of 12 months from manufacture date. Sample collection devices, crucial to the testing process, must be obtained separately and are not included in the kit.