STANDARDu2122 Q HIV 1/2 Ab 3-Line Test Kit: Rapid, Reliable Early HIV Diagnosis Tool
ProcurenetShort description
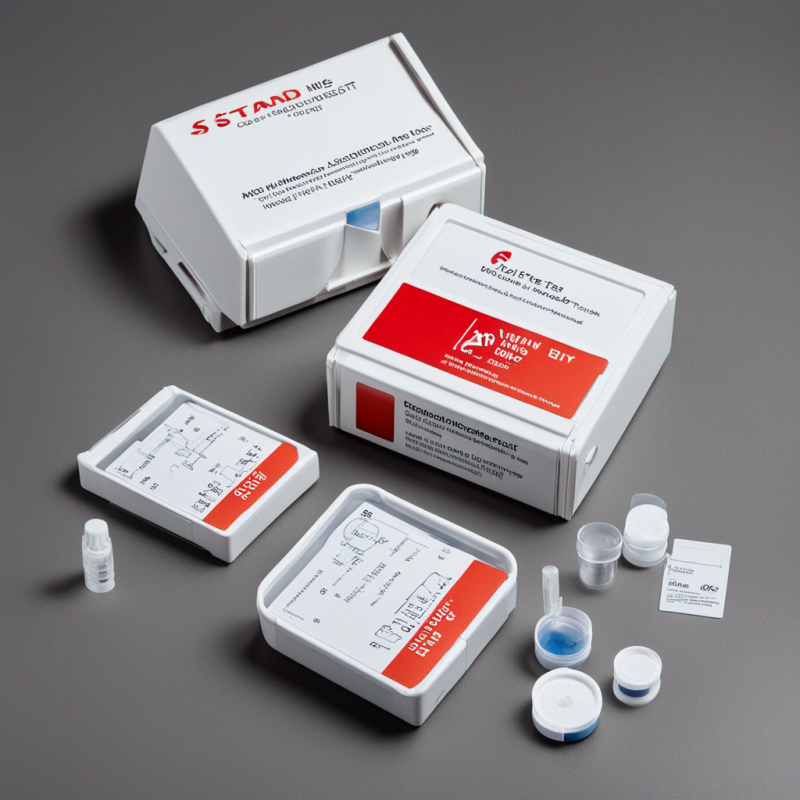
The STANDARD™ Q HIV 1/2 Ab 3-Line Test Kit is a sophisticated diagnostic tool, developed for rapid HIV detection in diverse human blood types – serum, plasma, venous, and capillary whole blood. Offering unrivaled precision and accuracy, this kit enables detection for both symptomatic and asymptomatic populations.
- Technology: Swift chromatographic immunoassay
- Kit Contents: 25 individually-packed devices, buffer bottle, instructions
- Result Time: Minimum of 10 minutes
Store between 2-40°C/36-104°F. Additional testing equipment not included. Check expiry date and storage conditions before use.
-
Procurenet Team Tshim Sha Tsui
Hong Kong 3 years
Description
STANDARDu2122 Q HIV 1/2 Ab 3-Line Test Kit: Revolutionize HIV Diagnostics
Embrace the new era of HIV diagnostics with the STANDARDu2122 Q HIV 1/2 Ab 3-Line Test Kit. Designed to deliver superior performance, this breakthrough kit offers qualitative detection of antibodies specific to both HIV-1 and HIV-2 in serum, plasma, venous, and capillary whole blood, becoming a reliable tool in combating the global HIV epidemic.
Unparalleled Features for Exceptional Results
- The robust Rapid Chromatographic Immunoassay technology ensures results within just 10 minutes, dramatically reducing patient anxiety related to waiting for outcomes.
- Unprecedented sensitivity allows optimal performance and reliable readings across diverse sample types like serum, plasma, venous, and capillary whole blood.
- Unmatched specificity aids in preventing false positives and negatives, ensuring the highest level of accuracy.
- Convenient test cassette format coupled with 25 easy-to-use tests per pack aid in efficient batch testing and stock management.
Note the Package Inclusions and Exclusions
Inclusions
- 25 individually packed test devices
- 1 Buffer Bottle (4 ml)
- 1 comprehensive user manual
Exclusions
- Various lab equipment
- Anti-coagulant tube
- Micropipette and tip
- Sterile lancet and swab
- Personal Protective Equipment
- Biohazard container
- Blood Collection Tool
Easy Storage and Long Shelf-Life
- Enjoy a 24-month shelf life for efficient inventory management.
- Temperature control is stress-free with a recommended storage temperature range between 2-40u00b0C/36-104u00b0F, under direct sunlight.
As a globally recognized medical tool, this kit holds its place in the WHO list of prequalified in vitro diagnostic products, affirming its trustworthiness and accuracy. The STANDARDu2122 Q HIV 1/2 Ab 3-Line Test Kit is the embodiment of advanced technology merging with clinical expertise, providing dependable results for early and accurate HIV diagnosis.
Experience the power of precision, speed, and reliability with the STANDARDu2122 Q HIV 1/2 Ab 3-Line Test Kit today.
Purchasing Notes
Bear in mind to check the expiry date and the completeness of the test device and the desiccant pack included in the pouch before use. Always read and adhere to the instructions in the manual.