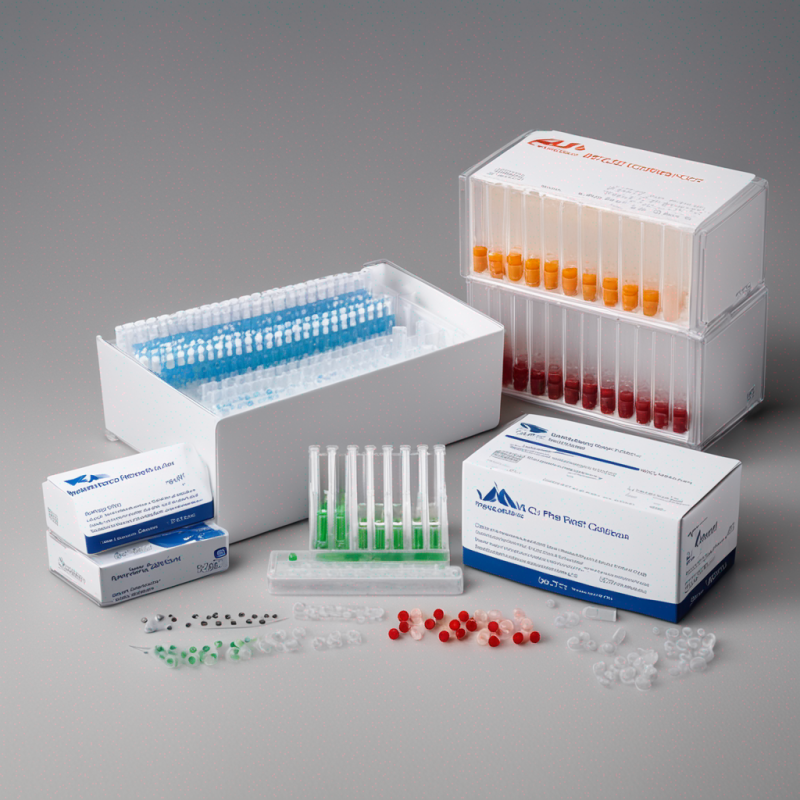
Premier Real-Time PCR Detection Kit for Genomic Research & Clinical Diagnostics
ProcurenetShort description
Enhance the precision of your genomic studies with the Premier Real-Time PCR Detection Kit. Designed for use in research, diagnostics, and healthcare, this kit excels in gene detection accuracy and sensitivity.
- High Sensitivity: Provides precise gene detection even at minimal concentrations, vital for detailed explorations.
- Speed and Reliability: Ensures rapid and dependable results, enabling increased workflow efficiency.
- Comprehensive Assembly: The kit incorporates a PCR reaction mix, an enzyme mix, and primer/probe sets, forming a versatile solution for an array of applications.
-
Procurenet Team Tshim Sha Tsui
Hong Kong 3 years
Description
Premier Real-Time PCR Detection Kit: Precision and Accuracy for Genomic Research and Clinical Diagnostics
With its leading-edge technology and proven efficacy, the Premier Real-Time PCR Detection Kit redefines the realm of genomic research and clinical diagnostics. This state-of-the-art kit enables remarkable accuracy and precision in amplifying and analyzing DNA sequences.
Sophisticated Precision for Reliable Results
Our distinguished PCR kit outperforms the competition by accurately working with minimal DNA samples. The kit's extreme specificity and sensitivity assure laser-sharp accuracy in every test, overcoming past constraints and traditional norms.
Simplified Diagnostics for Complex Requirements
The Premier Real-Time PCR Detection Kit streamlines the complicated process of molecular diagnostics. This breakthrough kit can identify a wide array of pathogenic bacteria and viruses more effectively than traditional methods. Its refined ability to detect minute amounts of DNA or RNA makes it a priceless tool for every laboratory.
- Inclusive of PCR reaction mix, enzyme mix, primers and probe sets.
- Positive and negative controls for complete accuracy.
- Provides an efficient, comprehensive testing solution.
Boost Your Lab Efficiency
Uniquely designed to provide results in real time, our PCR kit ensures high operational efficiency and reduces error occurrences. Its unwavering stability contributes to enhanced accuracy, propelling your lab productivity to new heights.
Unparalleled Performance Across Applications
Whether deployed for clinical diagnostics or genomic research, the Premier Real-Time PCR Detection Kit excels. Its unmatched precision and reliability make it a trusted solution in diverse research and diagnostic applications.
Embark on a journey of innovation in genomics and clinical diagnostics with our Premier Real-Time PCR Detection Kit. Experience unprecedented accuracy and precision to elevate your research and lab productivity.